10850
0
Where Are We In The Coronavirus Vaccine?
Where are we in the coronavirus vaccine? When will the distribution and vaccination take place?
Yazar: Eylem Özer
Yayınlanma: 23 Kasım 2020 15:09
Güncellenme: 2 Mart 2026 16:24
Where Are We In The Coronavirus Vaccine?
Where are we in the coronavirus vaccine? When will the distribution and vaccination take place?
Coronavirus (Covid-19) cases are increasing rapidly all over the world. According to the information on the website “Worldometer”, the number of people infected with coronavirus worldwide has exceeded 58.6 million, and 1 million 393 thousand 678 people died due to the coronavirus.
While the number of cases is increasing on the coronavirus front, positive news continues to come from the vaccine front. Speaking to CNN television, Dr. Moncef Slaoui said that the vaccine could be launched in the US on December 11. Slaoui reported that they aim to transport the vaccine to the areas where the vaccine will be administered within the first 24 hours after the vaccine is approved. Stating that they can start the application within 1-2 days after the vaccine is approved, Slaoui gave information about which groups will be prioritized in the vaccine.
Slaoui said that vaccines will be distributed according to the population of each state, and the states will decide who should be vaccinated first. It is estimated that priority in vaccination will be given to risk groups such as the elderly and healthcare workers. Slaoui noted that by this means, 70 percent of the population in the US can gain herd immunity as of May.
Good News From Companies
An application was made to the US Food and Drug Administration (FDA) last Friday for the approval of the coronavirus vaccine produced in partnership with Pfizer and BioNTech. For the vaccine, which has proven to be 95 percent effective, the FDA will discuss whether it will be approved on December 10. If the vaccine is approved, it is planned to produce 50 million doses by the end of the year.
The company called Moderna recently announced that the vaccine they produced was 95 percent effective, and announced that they expected approval in the coming weeks.
The latest news came from the Oxford-Astrazeneca vaccine. The vaccine developed in partnership with Oxford-Astrazeneca was reported to be 70 percent effective against coronavirus.
In addition, the US Drug and Food Administration (FDA) gave emergency approval for the experimental drug used in the treatment of US President Donald Trump to be used in the treatment of patients. The drug, produced by a biotechnology company called Regeneron, consists of the administration of two strong antibodies in the form of a cocktail. In the first studies on the drug, it was observed that the infection was under control and the rate of hospitalization was significantly reduced if the drug was administered in the early stages of the disease.
In addition, a similar treatment method developed by the pharmaceutical company Eli Lilly was approved in early November.
However, it is worth mentioning; The approval given by the FDA to Regeneron's treatment was surrounded by some limits. Namely, this treatment can be applied to patients who have a positive coronavirus test and are likely to get worse.
İLGİLİ HABERLER

European stocks soared and focus shifted to German retail sales after Powell's speech!

Forex Signal For TRY/USD: Inflation Slowdown in November.

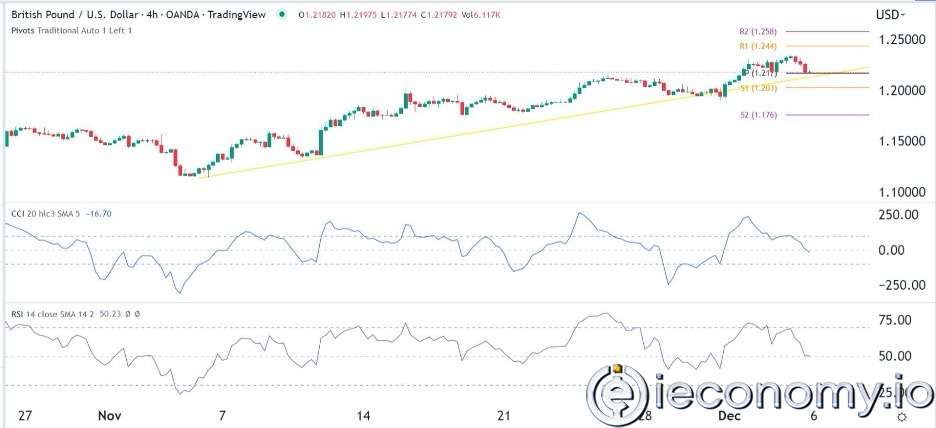
Forex Signal For GBP/USD: Bullish Trend Still Not Breaking While Recovery Continues.

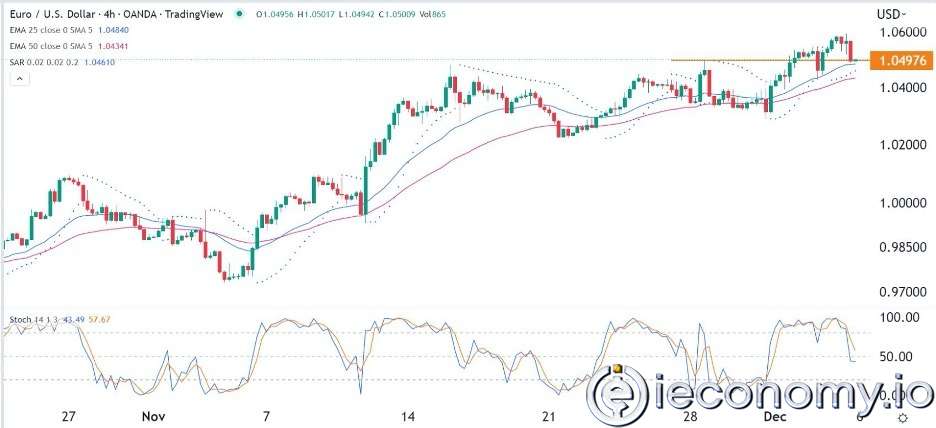
Forex Signal For EUR/USD: Starry US Data Points to Higher Fed Increases.

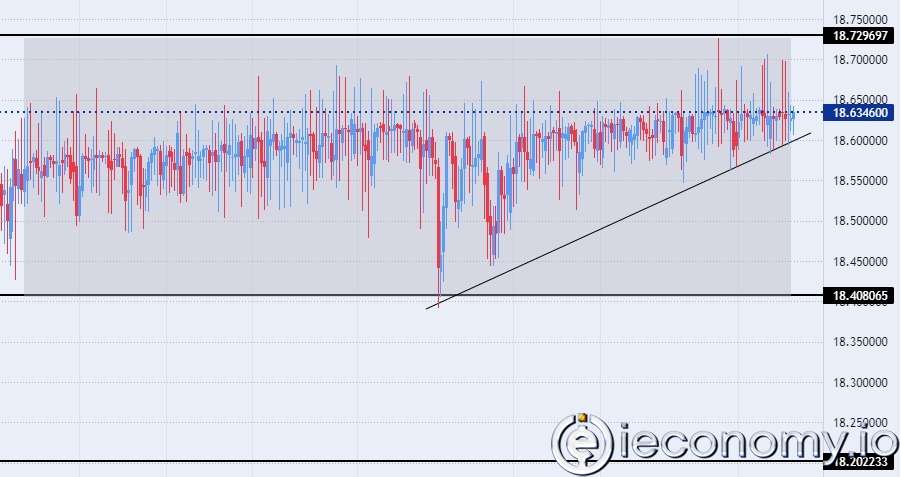
Forex Signal For BTC/USD: Downside Continues as Bitcoin Recovery Moves Less.
En Popüler Haberler
Yorum Yap
Yorumlar
Henüz yorum yapan yok! İlk yorumu siz yapın...