1555
0
Curevac has terminated the contract with Wacker Chemie
The Tübingen-based biotech company Curevac has terminated the contract with Wacker Chemie for the production of its Covid-19 vaccine.

Yazar: Tom Roberts
Yayınlanma: 15 Eylül 2021 06:05
Güncellenme: 3 Mart 2026 01:30
Curevac has terminated the contract with Wacker Chemie
The Tübingen-based biotech company Curevac has terminated the contract with Wacker Chemie for the production of its Covid-19 vaccine. The reason for this is a low need for production capacities for the mRNA vaccine candidate CVnCOV against Covid-19 from Curevac, as Wacker Chemie AG announced. However, the Munich-based MDax Group announced that there are no significant influences on sales and earnings in 2021 in the affected Wacker Biosolutions division. "That doesn't change anything about the medium-term goals for the Biosolutions division," said division manager Susanne Leonhartsberger. "We are confident that in the future we will be able to make the capacities freed up for other customers to produce their mRNA or other molecules." In June, Wacker announced that the mRNA platform was a product platform that could also be used for other mRNA vaccine candidates or other mRNA applications. Wacker and Curevac announced their cooperation agreement in November. This stipulated that Wacker should start producing the mRNA active substance for the Curevac vaccine at the Amsterdam site in the first half of 2021 as soon as it is approved. According to the information provided at the time, more than 100 million doses of the vaccine per year were planned, and an expansion was possible. However, Curevac has published disappointing data on the vaccine candidate's effectiveness. In an interim analysis of an approval-relevant clinical study with 40,000 test subjects, the agent had only achieved an effectiveness of 47 percent against Covid-19 diseases of any severity and thus did not meet the specified statistical success criteria. The vaccine candidate is currently being examined by the EMA. Curevac has now terminated the existing contracts with Wacker for the production of the active mRNA and with Celonic for the production and formulation of the active mRNA. Curevac's existing contracts with Rentschler Biopharma and Novartis for the production and formulation of mRNA are not affected and will remain in place. According to the information, the capacity adjustment will not limit the availability of clinical material for CV2CoV, the second-generation COVID-19 vaccine candidate developed jointly with GSK. A clinical trial for CV2CoV is expected to begin in the fourth quarter of 2021, according to Curevac.İLGİLİ HABERLER

European stocks soared and focus shifted to German retail sales after Powell's speech!

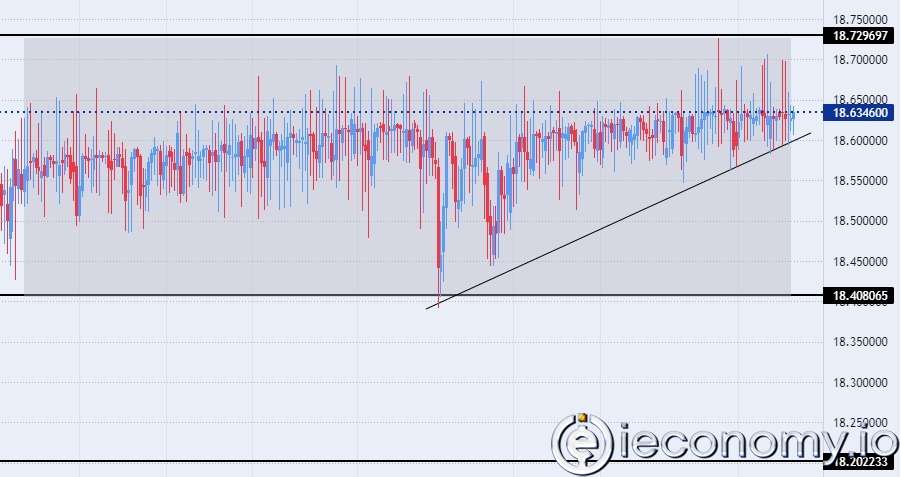
Forex Signal For TRY/USD: Inflation Slowdown in November.

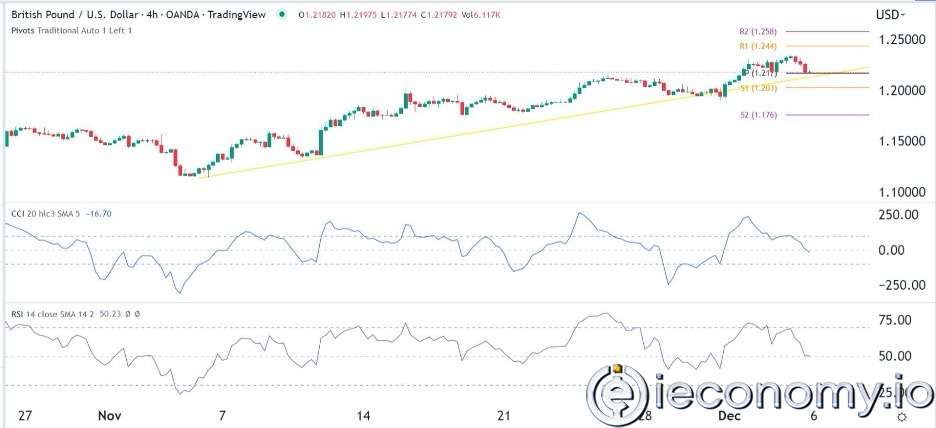
Forex Signal For GBP/USD: Bullish Trend Still Not Breaking While Recovery Continues.

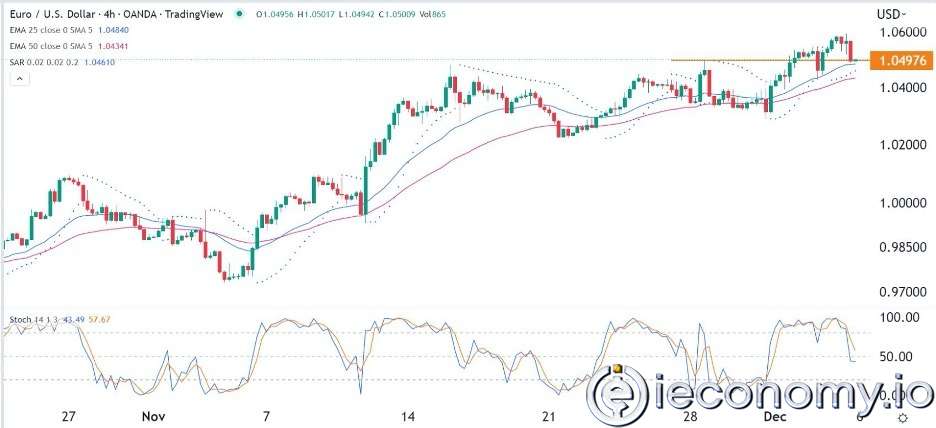
Forex Signal For EUR/USD: Starry US Data Points to Higher Fed Increases.

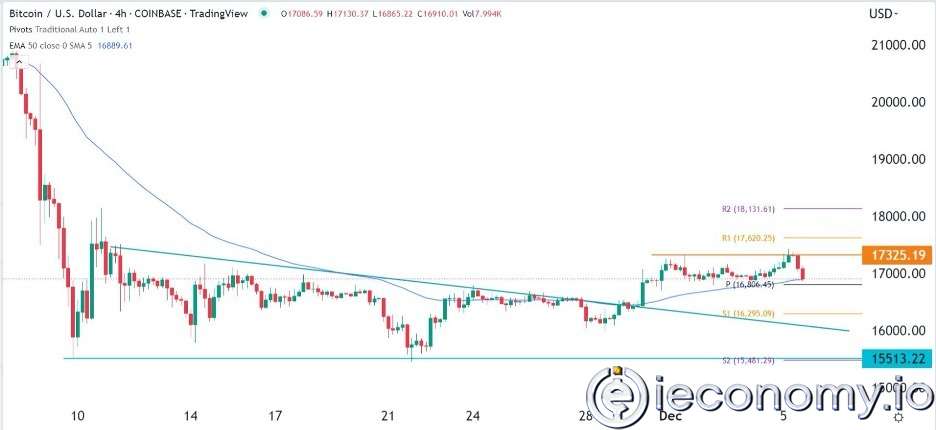
Forex Signal For BTC/USD: Downside Continues as Bitcoin Recovery Moves Less.
En Popüler Haberler
Yorum Yap
Yorumlar
Henüz yorum yapan yok! İlk yorumu siz yapın...