2939
0
Moderna Applies For Emergency Approval
In the race for a vaccine against the corona virus, the US company Moderna seems to be ahead. According to his own statements, he has applied for emergency approval in the USA - and also in the EU.
Yazar: Tom Roberts
Yayınlanma: 1 Aralık 2020 05:20
Güncellenme: 2 Mart 2026 21:47
Moderna Applies For Emergency Approval
As announced, the US pharmaceutical company Moderna was the first company to apply for approval for a corona vaccine in the EU. A corresponding application had been submitted to the European Medicines Agency Ema, confirmed a Moderna spokeswoman. At the same time, an application was made to the US FDA for emergency approval. The company announced that the relevant data had been sent to the FDA. The FDA then scheduled an advisory committee meeting for December 17 to discuss the application. Moderna had already announced on Monday morning that it wanted to apply for approvals on the same day. The Mainz-based manufacturer Biontech, together with the US company Pfizer, applied for emergency approval in the USA around two weeks ago. Moderna's preparation is comparatively similar to the Biontech / Pfizer vaccine in terms of its mode of action and its effectiveness.İLGİLİ HABERLER

European stocks soared and focus shifted to German retail sales after Powell's speech!

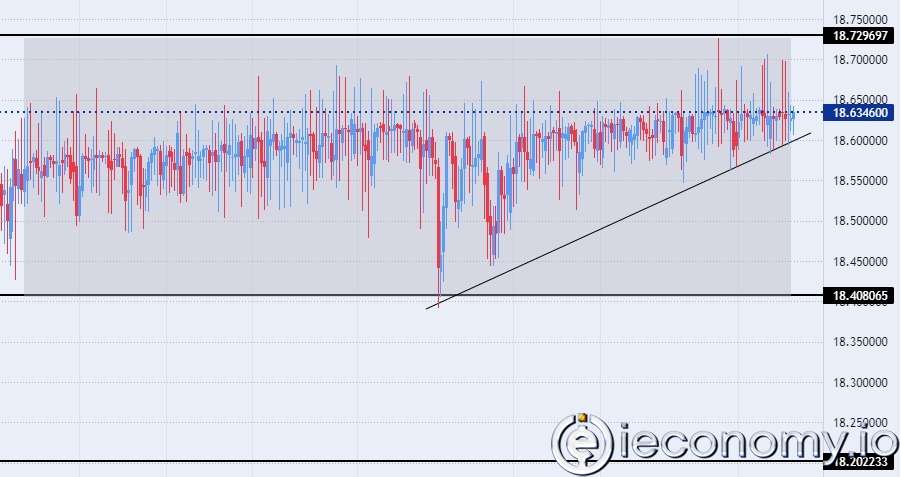
Forex Signal For TRY/USD: Inflation Slowdown in November.

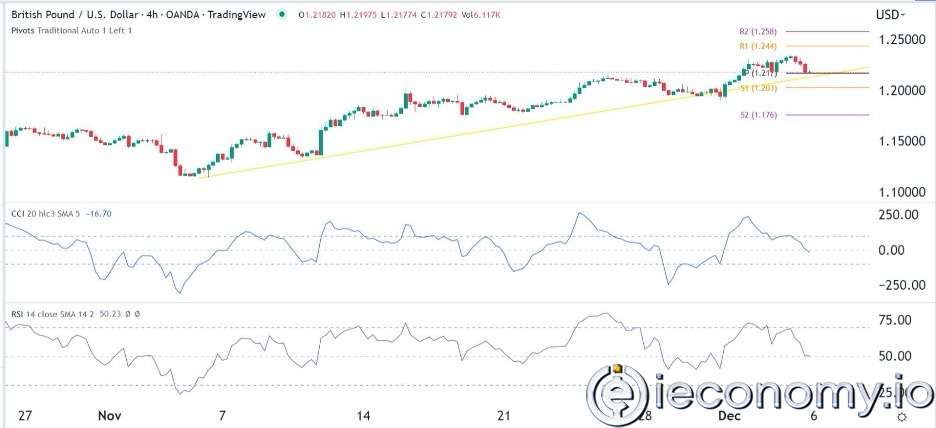
Forex Signal For GBP/USD: Bullish Trend Still Not Breaking While Recovery Continues.

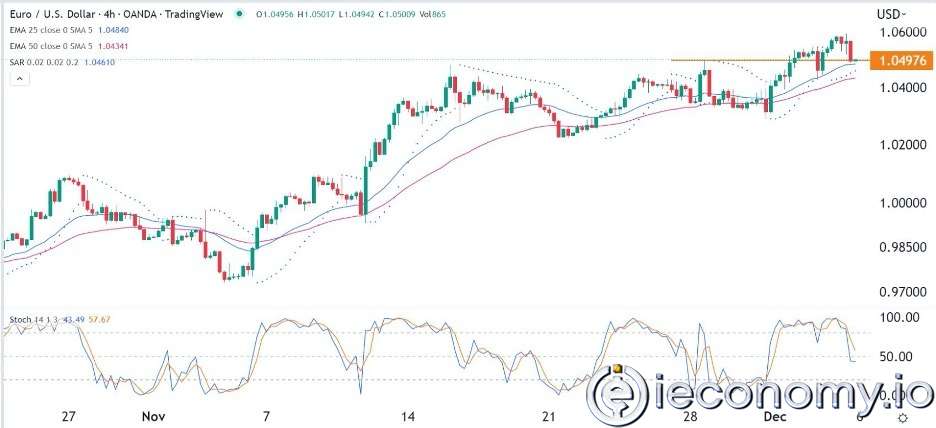
Forex Signal For EUR/USD: Starry US Data Points to Higher Fed Increases.

Forex Signal For BTC/USD: Downside Continues as Bitcoin Recovery Moves Less.
En Popüler Haberler
Yorum Yap
Yorumlar
Henüz yorum yapan yok! İlk yorumu siz yapın...